They lie like in a bee comb, each in its own micro compartment: hundreds of early synthetic human embryos. No egg or sperm cell was involved in their creation. They are made from the skin cells of a British woman, cells that have been programmed back into stem cells that can grow into all possible tissues.
These stem cells have organized themselves into a clump of cells, just like an embryo during pregnancy. They have formed a layer of other cells, like a vesicle around the embryo, which can form a placenta. And there is even a third layer of cells, the yolk sac, which provides an embryo with nutrition in the first weeks. All thanks to the right cocktail of signaling molecules and growth-promoting substances in their bee comb.
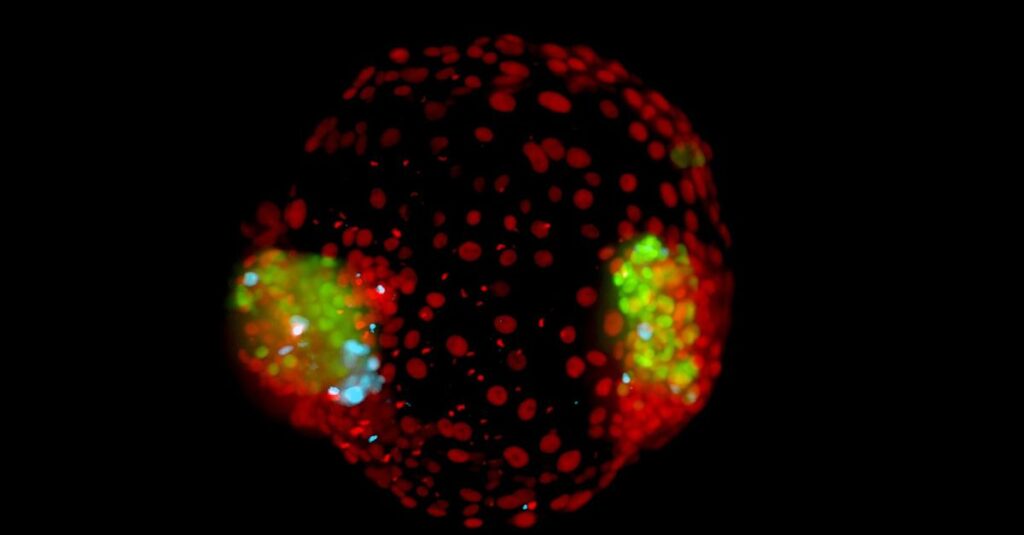
And as if this feat wasn’t enough: one in five micro-boxes contains an artificial identical twin.
Are they real embryos? No. Blastoids, the scientists at MUMC+ and the MERLN Institute in Maastricht call them. Embryo-like structures. And they are not intended to breed large numbers of new humans. They are models. “For the first time, this makes it possible to study how identical twins develop in those very early days,” says project leader and biomedical engineer Erik Vrij during a tour of the institute. “We can use this to learn what is needed for healthy implantation in the uterus, and thus improve IVF treatments, for example.”
Transparent plastic
The hundreds of embryos can be seen under a microscope in one of the MUMC+ labs. And anyone who does not look through the eyepieces of the microscope but looks underneath with the naked eye realizes that there are almost a hundred times more. All those living cell clumps are only in one of the 96 wells of a handy culture plate made of transparent plastic.
That record is an important asset of the institute. The living embryos can be filmed in this for days to chart their development. The cells that form the embryo are marked using a molecular technique in such a way that they emit green light under a fluorescent microscope, and the cells that form the placenta glow red. The plate is an invention of Vrij’s colleague, Stefan Giselbrecht. On April 10, together with colleagues, they published their scoop, identical twin embryos, in a scientific journal Advanced Materials.
“We can screen thousands of embryos this way,” says Vrij. “We look, among other things, at the effect of toxic substances and chromosomal abnormalities. The large numbers of embryos allow us to do good statistical analyses.”
In 2018, the researchers at the MERLN institute were the first in the world to create a blastoid, an artificial embryo from mouse stem cells. In the years that followed, one research group after another came up with such model embryos, not only from mice, but also from monkeys. And in June 2023, four research groups in the United Kingdom, Israel, the United States and China were unsure how quickly they each had to publish their results with human artificial embryos.
And now there are the twin embryos. “How identical twins came about was a mystery until now,” says Vrij. “Somewhere during development, the clump of cells in a fertilized egg splits into two clumps. In a third of identical twins, each embryo gets its own placenta and amniotic sac, but more often they share the placenta. Complications occur more often.” Scientists suspected that the moment at which the division takes place determines whether or not the placenta is divided. With the Maastricht artificial twin embryos, Vrij discovered the exact time and the process that plays a role.
Crucial for the development of the placenta is proper implantation of the embryo in the mucous membrane of the uterus, the endometrium. To study this, Vrij and his colleagues devised an ‘implantation chip’. At the bottom of a culture container they grow a layer of endometrial cells from a donor or a patient. Then they add a hundred blastoids. “After two days we rinse carefully and count how many embryos are released. This way we know how many have been properly nested.” The researchers use this to test, for example, which substances and hormones promote or hinder implantation. “The first IVF treatment is only successful in 35 percent of women, after three treatments this is 60 percent,” says Vrij. In the future, the best conditions for implantation could be sought personally for each woman.
Thousands at a time
The question on everyone’s lips is: can the cultured embryos grow into a real embryo? Not at the moment, says Vrij. “The development stops after two weeks, for reasons that are still unclear. But every embryo is just a little bit different. And we breed them by the thousands. Theoretically, there would be one that has exactly the right qualities for continued growth.” In any case, that will not fit into the micro compartments. But the mouse model embryos have already reached a stage comparable to mid-pregnancy, says Vrij. “They already have a beating heart and all kinds of organs in development.”
And artificial monkey embryos lead to a pregnancy – within a few days – once they are placed in the uterus.
The rapid developments surrounding human artificial embryos naturally raise ethical questions. MERLN scientists work closely with ethicists from MUMC+. For example, they conduct discussions with groups of people about the question of whether you can call a beating group of cells a heart. These occur in real human embryos approximately 21 days after fertilization.
The artificial embryos stop growing within fourteen days after fertilization. That is also the age limit up to which it is permitted to allow real human embryos from IVF clinics to continue to grow, according to the current Embryo Act.
Since 2022, a new bill has been in the works, which will include embryo-like structures (ELS). The same rules should apply to this as to real human embryos. But until that law is in place, “we are in principle still allowed to make anything with this technology,” says Vrij.
Also read
NRC editor Laura Wismans had twins and started looking for the genetics behind them
/s3/static.nrc.nl/bvhw/files/2022/10/data91846468-6c5aaf.jpg)